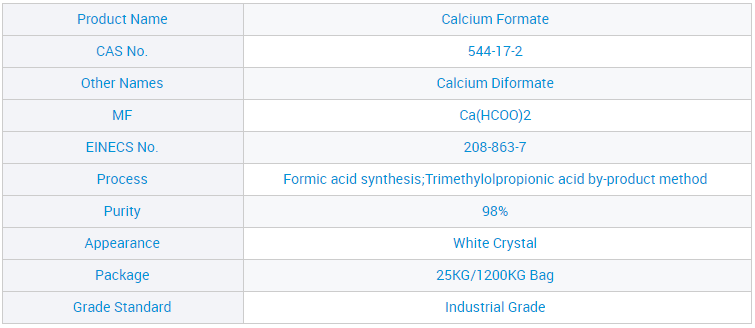
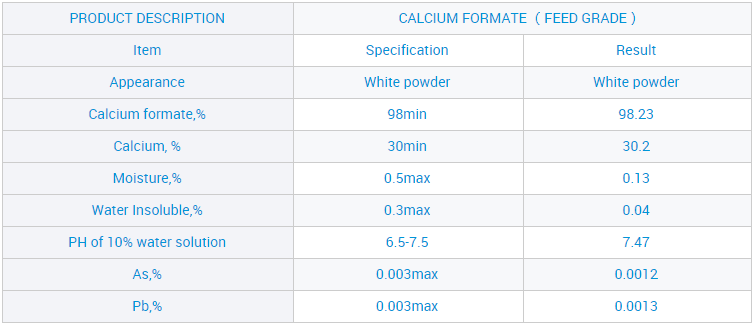
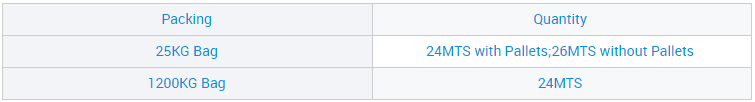
Samar da OEM Ciyarwar Jumla Mai Matsayin Calcium CF 98% don Ginawa
Kullum muna da yakinin cewa halin mutum yana yanke hukunci kan ingancin samfura, cikakkun bayanai suna yanke hukunci kan ingancin samfura, gami da MAI KYAU, MAI KYAU DA KYAU ga ƙungiyar OEM Supply Jumla Ciyarwar Kalori CF 98% don Ginawa, Da fatan za a aiko mana da bayanai da buƙatunku, ko ku ji daɗin tuntuɓar mu idan kuna da wasu tambayoyi ko tambayoyi da za ku iya yi.
Kullum muna da yakinin cewa halin mutum yana yanke hukunci kan ingancin kayayyaki, cikakkun bayanai suna yanke hukunci kan ingancin kayayyaki, tare da ruhin ma'aikata na gaske, masu inganci da kirkire-kirkire, tare da kewayon kayayyaki masu kyau, inganci mai kyau, farashi mai ma'ana da ƙira mai salo, ana amfani da kayayyakinmu sosai a wuraren jama'a da sauran masana'antu. Masu amfani suna da amincewa da kayayyakinmu sosai kuma suna iya biyan buƙatun tattalin arziki da zamantakewa masu tasowa akai-akai. Muna maraba da sabbin abokan ciniki daga kowane fanni na rayuwa don tuntuɓar mu don hulɗar kasuwanci ta gaba da cimma nasara a juna!










 Hanyoyin Shirya Tsarin Calcium
Hanyoyin Shirya Tsarin Calcium
Ana iya shirya sinadarin calcium ta hanyar amfani da hanyoyi masu zuwa:
Maganin hana shiga tsakani: React formic acid tare da mahaɗan calcium (kamar calcium hydroxide ko calcium carbonate) don samar da sinadarin calcium form. Lissafin amsawar shine:
Ca(OH) 2 +2HCOOH→Ca(HCOO) 2 +2H2O
Amsar sinadarin calcium hydroxide da acetic acid: Da farko, a yi maganin calcium hydroxide da acetic acid, sannan a yi maganin sodium formate don a sami sinadarin calcium. Daidaito tsakanin martanin sune:
Ca(OH) 2 +2CH3 COOH→Ca(CH3 COO) 2 +2H2O
Ca(CH3 COO) 2 +2HCOONa→Ca(HCOO) 2 +2CH3 COONa















