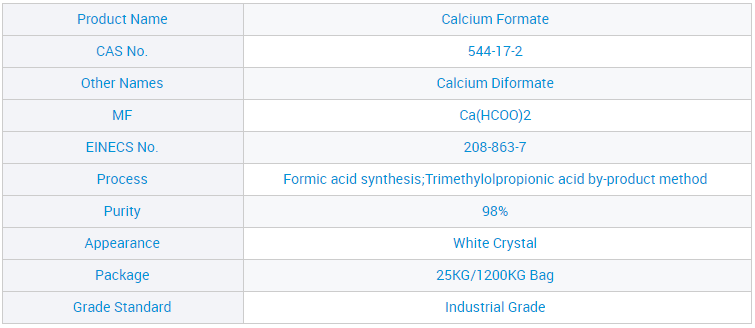
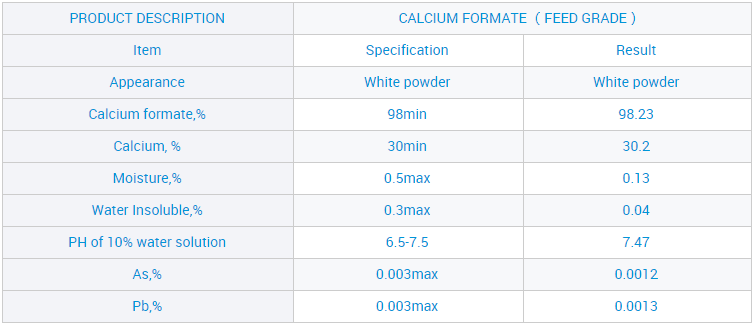
Ƙwararrun Masana'antar CAS: 544-17-2 Farashin Masana'anta Calcium Form 98% don Ciyarwa C2H2O4ca
Muna jin daɗin kyakkyawan matsayi tsakanin masu siyanmu saboda kyawun kayanmu, inganci mai kyau, farashi mai tsauri da kuma babban tallafi ga ƙwararrun masana'antar Sinawa CAS: 544-17-2 Farashin Masana'antu Calcium Format 98% don Ciyarwar C2H2O4ca, Bayan shekaru 10 na ƙoƙari, muna jawo hankalin abokan ciniki ta hanyar farashi mai kyau da kyakkyawan sabis. Bugu da ƙari, gaskiya da gaskiya ne muke taimaka mana koyaushe mu zama zaɓin farko na abokan ciniki.
Muna jin daɗin kyakkyawan matsayi tsakanin masu siyanmu saboda kyawun kayanmu, inganci mai kyau, farashi mai tsauri da kuma babban tallafi ga. Mun shafe sama da shekaru 20 muna yin kayayyakinmu. Galibi muna yin su ne a cikin jimilla, don haka yanzu muna da farashi mafi kyau, amma mafi inganci. A cikin shekarun da suka gabata, mun sami ra'ayoyi masu kyau, ba wai kawai saboda muna samar da mafita masu kyau ba, har ma saboda kyakkyawan sabis ɗinmu na bayan-sayarwa. Muna nan muna jiran ku don tambayar ku.










 Hanyoyin Haɗa Calcium
Hanyoyin Haɗa Calcium
Hanyar Calcium Carbonate
Daidaito na Amsawar Calcium:
CaCO3+2HCOOH=Ca(HCOO)2+CO2↑+H2O
Aiki: Ƙara wani adadin formic acid a cikin tankin amsawa; a hankali a ƙara sinadarin calcium carbonate mai ƙarfi yayin da ake juyawa (haɗarin yana ci gaba da ƙarfi, yana fitar da adadi mai yawa na CO2
da kuma zafi). Rabon sinadarin formic acid zuwa calcium carbonate shine 1.1:1.0. Idan amsawar ta kusa kammala, sai a zuba madarar lemun tsami don daidaita pH zuwa 7-8, a ajiye a wuta na tsawon awanni 1-2, sannan a zuba ruwan sulfide don cire ions masu nauyi na ƙarfe. Bayan tacewa, a tattara tacewa don yin crystallization, a yi amfani da centrifuge, sannan a busar da shi don samun samfurin (ana sake yin amfani da giyar uwa).














